Views: 222 Author: Lake Publish Time: 2026-01-24 Origin: Site








Content Menu
● The Japanese Advantage in Medical Device Manufacturing
>> 1.1 Cultural Commitment to Quality and Precision
>> 1.2 Rigorous Regulatory Environment
>> 1.3 Integrated Supply Chain Ecosystem
● Key Japanese Video Laryngoscope Manufacturers and Suppliers
>> 2.1 Major Integrated Medical Corporations
>> 2.2 Specialized Airway Management Companies
>> 2.3 Emerging Technology Innovators
● Technological Innovations from Japanese Manufacturers
>> 3.1 Advanced Optical Systems
>> 3.2 Ergonomic Design Excellence
>> 3.3 Integration and Connectivity Solutions
● The OEM/ODM Partnership Model with Japanese Suppliers
>> 4.1 Benefits of Partnering with Japanese Manufacturers
>> 4.2 Customization Capabilities
● Market Trends and Future Directions
>> 5.1 Increasing Adoption in Diverse Clinical Settings
>> 5.2 Technological Convergence
>> 5.3 Global Market Expansion Strategies
● Frequently Asked Questions (FAQ)
>> 1. What distinguishes Japanese video laryngoscope manufacturers from other global suppliers?
>> 2. How do Japanese manufacturers ensure product quality and reliability?
>> 3. Can international hospitals purchase directly from Japanese manufacturers?
>> 4. What support services do Japanese manufacturers typically provide?
>> 5. How are Japanese manufacturers addressing sustainability concerns?
The Japanese medical device market stands as a global benchmark for precision, innovation, and quality. Within this sophisticated landscape, the video laryngoscope sector has emerged as a critical domain, revolutionizing airway management practices worldwide. For international medical brands, hospital procurement departments, and healthcare distributors, identifying reliable video laryngoscope manufacturers and suppliers in Japan represents a strategic opportunity to access cutting-edge technology built on decades of engineering expertise. This comprehensive guide explores the Japanese video laryngoscope ecosystem, examining key players, technological innovations, market dynamics, and the strategic advantages of partnering with Japanese manufacturers.

Japanese manufacturing is renowned for its unwavering dedication to quality, precision, and continuous improvement. This cultural foundation, deeply rooted in principles like *kaizen* (continuous improvement) and *monozukuri* (the art of making things), creates an environment where video laryngoscope manufacturers and suppliers in Japan consistently produce devices of exceptional reliability and performance. The meticulous attention to detail extends from initial design through production and quality control, resulting in products that healthcare professionals worldwide have come to trust in critical airway management situations.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) maintains one of the world's most stringent regulatory frameworks for medical devices. This rigorous oversight ensures that all video laryngoscope manufacturers and suppliers operating in Japan adhere to exacting safety and efficacy standards. For international partners, this regulatory rigor translates to confidence in product quality, reduced liability concerns, and smoother regulatory pathways in their own markets when sourcing from Japanese suppliers.
Japan boasts a highly integrated and specialized supply chain for medical device components. From precision optics manufacturers and microelectronics specialists to advanced polymer producers, this ecosystem enables video laryngoscope manufacturers and suppliers in Japan to maintain exceptional quality control throughout the production process. This vertical integration allows for superior coordination, faster innovation cycles, and consistent component quality that is difficult to achieve in more fragmented manufacturing environments.
Several of Japan's largest medical technology companies have established significant presence in the video laryngoscope market. These corporations leverage extensive research and development resources, global distribution networks, and multidisciplinary expertise to develop comprehensive video laryngoscope solutions. When evaluating video laryngoscope manufacturers and suppliers in Japan from this category, partners can expect:
- Comprehensive product ecosystems integrating video laryngoscopes with monitoring systems and medical record interfaces
- Extensive clinical validation and research supporting product efficacy
- Global regulatory expertise and support for market entry
- Robust after-sales service and technical support networks
A dedicated segment of Japanese manufacturers focuses exclusively on airway management devices, bringing specialized expertise to video laryngoscope development. These video laryngoscope manufacturers and suppliers typically offer:
- Deep clinical understanding of airway management challenges
- Innovative designs specifically addressing difficult airway scenarios
- Lightweight and ergonomic devices optimized for prolonged use
- Specialized training programs and educational resources for users
Japan's vibrant startup ecosystem has produced several innovative companies pushing the boundaries of video laryngoscope technology. These emerging video laryngoscope manufacturers and suppliers in Japan often introduce:
- Novel optical systems and imaging technologies
- Advanced materials improving durability and patient safety
- Integrated artificial intelligence for procedural guidance
- Connectivity solutions enabling telemedicine applications

Japanese optical engineering expertise directly benefits video laryngoscope technology through:
- High-definition imaging with exceptional clarity and color accuracy
- Anti-fogging technologies ensuring consistent visualization
- Wide-angle lenses providing comprehensive airway views
- Low-light performance optimization for emergency settings
Human factors engineering represents a particular strength of video laryngoscope manufacturers and suppliers in Japan, resulting in:
- Lightweight designs reducing clinician fatigue during prolonged procedures
- Intuitive control interfaces minimizing the learning curve
- Balanced weight distribution enhancing procedural control
- Durable construction withstands repeated sterilization cycles
Modern video laryngoscopes from Japanese manufacturers increasingly feature:
- Wireless connectivity for unencumbered movement
- Integration with hospital information systems
- Recording capabilities for documentation and training
- Telemedicine compatibility for remote expert consultation
International brands and healthcare organizations choosing to work with video laryngoscope manufacturers and suppliers in Japan through OEM (Original Equipment Manufacturer) or ODM (Original Design Manufacturer) arrangements typically gain:
- Access to world-class engineering and manufacturing expertise
- Reduced time-to-market for new products
- Shared regulatory compliance expertise
- Cost efficiencies through established supply chains
- Enhanced product credibility through "Made in Japan" association
Japanese manufacturers offer extensive customization options including:
- Brand-specific housing and interface designs
- Customized blade sizes and configurations
- Tailored software interfaces and features
- Market-specific regulatory compliance adaptations
- Packaging and labeling aligned with brand identity
Video laryngoscopes from video laryngoscope manufacturers and suppliers in Japan are finding applications beyond traditional operating rooms in:
- Emergency departments and pre-hospital care
- Intensive care units and critical care transport
- Military and disaster response medicine
- Veterinary medicine applications
Future developments from Japanese manufacturers are likely to include:
- Integration with artificial intelligence for procedural guidance
- Augmented reality overlays providing anatomical guidance
- Miniaturization for enhanced portability
- Advanced materials reducing costs while maintaining quality
Japanese video laryngoscope manufacturers and suppliers are increasingly pursuing:
- Strategic partnerships with international distributors
- Localized product adaptations for specific markets
- Educational initiatives to promote proper utilization
- Outcome research demonstrating clinical efficacy
The Japanese video laryngoscope manufacturing sector represents a convergence of technological excellence, precision engineering, and clinical understanding that is difficult to match globally. For healthcare providers, procurement specialists, and international medical brands, engaging with video laryngoscope manufacturers and suppliers in Japan offers access to devices that embody reliability, innovation, and thoughtful design. As airway management continues to evolve with technological advancements, Japanese manufacturers are well-positioned to lead through their commitment to quality, their innovative capabilities, and their understanding of clinical needs. The strategic advantages of partnering with these manufacturers extend beyond product acquisition to encompass collaborative development, regulatory support, and shared commitment to improving patient outcomes through superior medical technology.
Contact us to get more information!
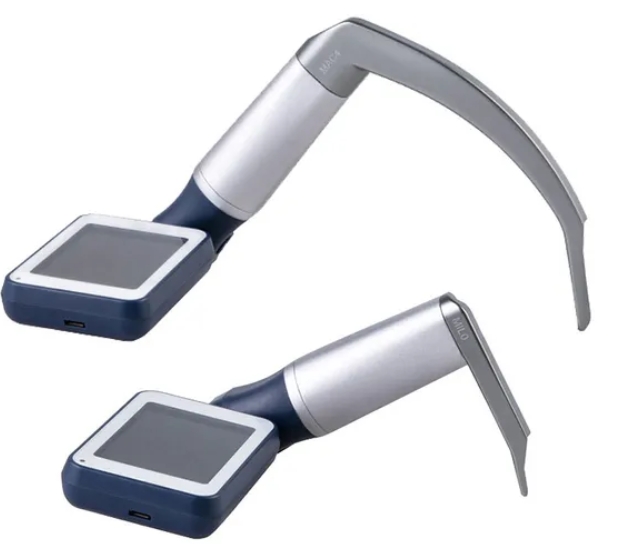
Japanese manufacturers are distinguished by their unparalleled commitment to precision engineering, rigorous quality control systems, and innovative approach to solving clinical challenges. The integration of advanced optics expertise, ergonomic design principles, and reliable electronics creates products renowned for their durability and performance in critical situations.
Quality assurance begins with component selection from trusted suppliers within Japan's integrated manufacturing ecosystem. Throughout production, multiple quality checkpoints, automated testing protocols, and meticulous inspection processes ensure every device meets exacting standards. Post-production, devices undergo functional testing and sterilization validation before release.
While some manufacturers maintain direct international sales channels, most work through authorized distributors or regional partners. These partnerships ensure proper regulatory compliance, localized support, and appropriate clinical training. For large healthcare systems, direct procurement relationships can sometimes be established with manufacturer approval.
Comprehensive support includes clinical training programs, technical support services, repair and maintenance options, and regulatory documentation assistance. Many manufacturers also provide educational resources, procedural guidelines, and access to clinical evidence supporting their products' efficacy.
Japanese manufacturers are implementing various sustainability initiatives including energy-efficient production processes, recyclable packaging materials, device refurbishment programs, and responsible end-of-life management options. Some are developing more durable devices with longer lifespans to reduce environmental impact.
[1] https://www.pmda.go.jp/english/
[2] https://www.japanmedicaldevice.com
[3] https://www.medtecjapan.com