Views: 222 Author: Lake Publish Time: 2026-01-29 Origin: Site








Content Menu
● The Indian Advantage in Disposable Medical Device Manufacturing
>> Cost-Competitive Production Ecosystem
>> Engineering Talent and Technical Capabilities
>> Regulatory Evolution and Quality Focus
● Key Indian Disposable Video Laryngoscope Manufacturers and Suppliers
>> Large, Diversified Medical Device Conglomerates
>> Specialized Airway Management and Anesthesia Companies
>> Emerging, Agile MedTech Innovators and Exporters
● Product Portfolio and Technological Features
>> Core Design and Functional Attributes
>> Differentiation and Innovation
● The OEM and Contract Manufacturing Proposition
>> Comprehensive Service Models for Global Brands
>> Supply Chain and Logistics Competence
● Market Focus and Global Reach
>> Dominance in Price-Sensitive and Emerging Markets
>> Penetration into Regulated Markets
● Challenges and Considerations for International Partners
>> Ensuring Consistent Quality
>> Navigating Logistics and Communication
>> Market Expansion and Strategic Alliances
● Frequently Asked Questions (FAQ)
>> 1. What is the typical price range for disposable video laryngoscopes from Indian manufacturers?
>> 3. Can Indian manufacturers provide fully customized disposable video laryngoscopes for my brand?
India has emerged as a pivotal global hub for medical device manufacturing, with its capabilities in producing high-quality, cost-effective disposable medical equipment gaining significant international recognition. Within this landscape, the market for disposable video laryngoscopes has witnessed remarkable growth, driven by increasing awareness of infection control, the rise of single-use device protocols, and the need for affordable advanced airway management tools in diverse healthcare settings. Indian video laryngoscope manufacturers and suppliers are strategically positioned to meet this global demand, leveraging the country's strengths in electronics assembly, precision plastics engineering, and large-scale manufacturing. This article provides a comprehensive analysis of India's leading disposable video laryngoscope manufacturers and suppliers, exploring their technological capabilities, market positioning, and the compelling value proposition they offer to international brands, distributors, and healthcare systems seeking reliable OEM partnerships and procurement sources.

India's primary strength in the global medical device market stems from its unparalleled cost efficiency, which extends comprehensively to the production of disposable video laryngoscopes. This advantage is multi-faceted:
Labor and Operational Costs: Compared to manufacturing bases in North America, Europe, or even East Asia, India offers significantly lower costs for skilled engineering labor and factory operations. This allows video laryngoscope manufacturers and suppliers in India to achieve very competitive price points without compromising the core functional integrity of the devices.
Scale and Vertical Integration: Many Indian manufacturers benefit from economies of scale, producing not only video laryngoscopes but also a wide range of other disposable medical products. This scale, combined with a growing domestic supply chain for electronic components, plastics, and packaging materials, creates a vertically efficient production environment. For international partners, this translates into stable pricing and reduced supply chain vulnerability.
Government Support and "Make in India" Initiative: The Indian government's "Make in India" campaign and Production Linked Incentive (PLI) schemes for medical devices actively encourage domestic manufacturing and exports. This policy framework provides financial and logistical support to video laryngoscope manufacturers and suppliers, enhancing their global competitiveness and enabling them to invest in better technology and quality systems.
India possesses a vast pool of English-speaking engineers specializing in electronics, software, and biomedical engineering. This talent directly feeds into the R&D and production teams of video laryngoscope manufacturers and suppliers. Their expertise is crucial for:
Miniaturization and Design: Developing compact, handheld disposable video laryngoscopes that integrate a camera, LED lights, power source, and often a display into a single, ergonomic unit.
Image Processing Software: Creating and refining the firmware and software algorithms that ensure clear, real-time video transmission with features like anti-fogging digital enhancement, which is critical for clinical use.
Design for Manufacturability (DFM): Engineering products that are not only clinically effective but also optimized for high-speed, low-cost assembly—a key requirement for disposable devices.
The Indian medical device regulatory landscape, governed by the Central Drugs Standard Control Organization (CDSCO), has undergone significant strengthening with the new Medical Devices Rules. Reputable Indian video laryngoscope manufacturers and suppliers now routinely comply with:
ISO 13485 Certification: The international standard for quality management systems specific to medical devices, which is a baseline for credible manufacturers.
CE Marking (for EU exports): Many export-oriented Indian firms design their disposable video laryngoscopes to meet the requirements of the European Union's Medical Device Regulation (MDR) from the outset.
US FDA Compliance: An increasing number of top-tier Indian manufacturers have US FDA 510(k) clearances for their devices, facilitating entry into the large and demanding North American market.
This regulatory maturation means international buyers can source from Indian video laryngoscope manufacturers and suppliers with greater confidence in product safety and efficacy.
Several established Indian healthcare giants have entered the disposable video laryngoscope segment, bringing significant resources and distribution networks:
Opto Circuits (India) Ltd / Cardiac Science Corporation: This group has a strong presence in patient monitoring and critical care devices. Their venture into disposable video laryngoscopes leverages their expertise in miniaturized medical electronics. They act as full-scale video laryngoscope manufacturers and suppliers, offering extensive OEM capabilities and boasting manufacturing facilities that are often US FDA and ISO 13485 certified, appealing to partners requiring rigorous compliance.
Hindustan Syringes & Medical Devices Ltd (HMD): A world leader in disposable syringes, HMD has successfully diversified into other single-use medical devices. Their foray into disposable video laryngoscopes capitalizes on their mastery of high-volume, precision plastics manufacturing and sterile packaging. They are formidable video laryngoscope suppliers, particularly for tenders and distributors requiring massive volumes at highly competitive prices with assured quality consistency.
A number of Indian firms focus specifically on equipment for anesthesia, emergency medicine, and respiratory care:
BPL Medical Technologies: With a heritage in medical electronics, BPL has developed a range of medical devices including disposable video laryngoscopes. They emphasize user-friendly design and reliability. As video laryngoscope manufacturers and suppliers, they often focus on creating value-engineered products suitable for clinics, ambulances, and hospitals in cost-sensitive markets, while maintaining export-quality standards.
Medi Devices Pvt. Ltd.: This company specializes in respiratory and anesthesia products. Their disposable video laryngoscope offerings are typically designed with direct input from anesthesiologists, focusing on form factors and blade geometries that address common clinical challenges. They serve as knowledgeable partners for OEM clients looking for clinically informed design input.
India's vibrant startup and SME sector has produced several nimble players:
Sinapss Medical: Representing the new generation of Indian video laryngoscope manufacturers and suppliers, companies like Sinapss often focus on innovative features, such as integrated recording or wireless connectivity, even in disposable formats. They are characterized by agility, rapid prototyping capabilities, and a strong focus on the export market from day one. They are ideal partners for brands seeking to launch feature-differentiated products or explore novel form factors.
Numerous Export-Oriented SMEs: Cities like Mumbai, Delhi, and Bengaluru host a cluster of small to medium-sized enterprises that have carved a niche as dedicated video laryngoscope suppliers to international markets. These firms often excel in providing highly customizable white-label and OEM services, offering flexibility that larger corporations sometimes cannot match. They are a prime source for distributors looking to establish their own branded line of disposable video laryngoscopes.
Disposable video laryngoscopes from Indian manufacturers typically encompass several key design philosophies:
All-in-One, Single-Use Design: The dominant model integrates a blade, a built-in CMOS camera with LED illumination, a battery, and a small integrated screen (or a connector for an external monitor/tablet) into a single sterile package. After use, the entire unit is discarded, eliminating any risk of cross-infection and the cost of reprocessing.
Ergonomics and Portability: Devices are designed to be lightweight, balanced, and easy to handle, often with anti-slip grips. Their disposable nature makes them ideal for code carts, emergency kits, and field use.
Image Quality: While cost-constrained, competitive products offer adequate VGA to HD resolution sufficient for clear glottic view. Advanced models may include wide-angle lenses and software-based image enhancement.
Leading Indian video laryngoscope manufacturers and suppliers are moving beyond basic functionality:
Blade Variety: Offering different blade styles (Macintosh, Miller) and sizes (from neonatal to large adult) even within disposable lines to cater to diverse patient populations and clinician preferences.
Connectivity Options: While most are standalone, some models offer optional wired or wireless (Bluetooth/Wi-Fi) connectivity to stream the video to larger external monitors, tablets, or for recording purposes.
Battery and Power Management: Ensuring the device is pre-powered and ready for immediate use with a battery life sufficient for multiple intubation attempts.
Packaging and Sterility: Utilizing robust, sterile barrier packaging (typically ethylene oxide or gamma radiation sterilized) that maintains integrity until the point of use.
India is exceptionally strong in the OEM/contract manufacturing space for disposable video laryngoscopes. Services include:
White-Label Manufacturing: Complete production of devices to be sold under the client's brand, with custom packaging, documentation, and labeling.
Custom Design and Development: Collaborative engineering to adapt an existing platform or develop a new product tailored to specific market requirements, feature sets, or price points.
Regulatory Support and Documentation: Assistance in preparing the technical file, test reports, and other documentation needed for regulatory submissions in the partner's target market (e.g., CE Technical File, FDA 510(k)).
Indian video laryngoscope manufacturers and suppliers have developed efficient export operations:
Estimated Cost Structure: They provide one of the world's most competitive landed costs for disposable devices, factoring in production, packaging, and freight.
Quality and Consistency at Scale: The ability to maintain quality across very large production runs is a hallmark of the top-tier Indian manufacturers, making them reliable partners for large distributors and institutional buyers.
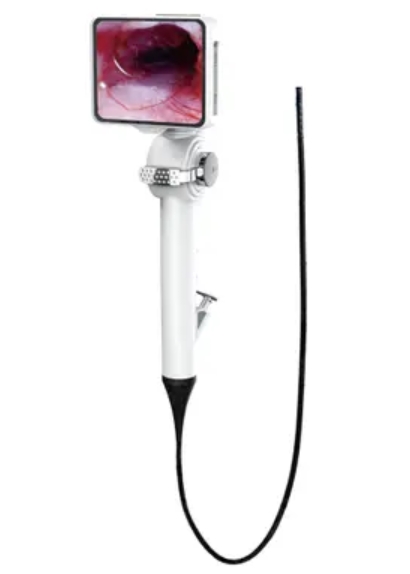
Indian-made disposable video laryngoscopes have found strong acceptance in markets where cost is a primary driver:
Public Health Systems and Tenders: Government procurement programs in Africa, Southeast Asia, the Middle East, and Latin America often source from Indian video laryngoscope suppliers due to their ability to meet quality thresholds at the lowest bid price.
Private Healthcare in Developing Nations: Hospitals and clinics in growing economies prioritize Indian devices for their balance of advanced technology and affordability.
Increasingly, high-quality Indian manufacturers are supplying to:
Europe: Leveraging CE marks and competitive pricing to supply distributors and group purchasing organizations (GPOs).
North America: Through FDA-cleared devices, Indian video laryngoscope manufacturers and suppliers are becoming a viable second-source or value-line option for US and Canadian markets.
Oceania and Other Regions: Meeting the specific regulatory standards of Australia (TGA), GCC countries, and others.
While the best Indian manufacturers are world-class, the market has variability. Due diligence is critical:
Factory Audits and Certifications: Verifying valid ISO 13485 certification and conducting on-site audits (virtual or physical) to assess quality systems, production cleanliness, and testing protocols.
Sample Evaluation and Clinical Testing: Always conducting thorough functional and, if possible, clinical evaluations of pre-production samples before committing to large orders.
Intellectual Property (IP) Protection: Establishing clear legal agreements regarding design ownership, confidentiality, and manufacturing rights is essential when engaging in co-development.
Lead Times and Shipping: Understanding total lead time from order to delivery at port, including production, quality checks, and ocean freight.
Cultural and Business Practices: Building strong, transparent communication channels and understanding Indian business negotiation styles can foster more successful long-term partnerships.
The trajectory points toward Indian video laryngoscope manufacturers and suppliers incorporating more advanced features:
Enhanced Imaging: Transition to consistently higher-resolution cameras and better low-light performance.
Smart Features: Integration of basic AI for anatomy recognition or procedural guidance, even in disposable formats.
Sustainability Initiatives: Exploration of more eco-friendly materials or partial recyclability in response to environmental concerns about single-use plastics, though this remains a complex challenge.
Deepening OEM Partnerships: Expect more strategic, long-term OEM alliances between Indian manufacturers and global medtech brands looking to optimize their cost structure.
Focus on Training and Education: Leading suppliers may bundle their devices with simulation software or training modules to increase adoption in new markets.
India stands as a global powerhouse for the manufacturing and supply of disposable video laryngoscopes. The country's unique combination of deep technical expertise, exceptional cost efficiency, scalable production capacity, and a rapidly maturing quality and regulatory framework has created an ecosystem of highly competitive video laryngoscope manufacturers and suppliers. For international medical brands, distributors, and healthcare providers, partnering with top-tier Indian firms offers a strategic path to accessing affordable, reliable, and clinically effective single-use airway visualization technology. While thorough due diligence in selecting the right partner is paramount, the opportunities presented by India's medtech sector are immense. As Indian video laryngoscope manufacturers and suppliers continue to innovate and align with the highest global standards, they are poised to play an increasingly central role in democratizing access to advanced airway management tools worldwide, improving patient safety and outcomes across diverse healthcare settings.
Contact us to get more information!

Indian video laryngoscope manufacturers and suppliers are known for offering some of the most competitive prices globally. For a basic, functional disposable unit with an integrated screen, prices can range from as low as $30 to $80 per unit in OEM quantities, depending on specifications, order volume, and included features (e.g., blade type, resolution, connectivity options). This cost advantage is a primary reason for sourcing from India, especially for high-volume procurement.
First, verify that the manufacturer holds valid ISO 13485 certification. Second, confirm the product has the necessary regulatory mark for your target market—CE Mark for Europe, FDA 510(k) number for the USA, or other local certifications. Third, request a detailed specification sheet and test reports (e.g., for electrical safety, biocompatibility, sterility). Finally, conduct a factory audit (or hire a third-party inspector) and rigorously test product samples before finalizing any agreement. Reputable Indian video laryngoscope manufacturers and suppliers will be transparent with this information.
Absolutely. Customization is a core strength of many Indian video laryngoscope manufacturers and suppliers. This can include: custom branding (logo, colors) on the device and packaging, modification of blade design or handle ergonomics, tailoring of software features or user interface, and development of specific packaging formats. The level of customization depends on the order volume and the manufacturer's capabilities, which should be discussed in detail during the quoting process.
Terms vary, but common structures include a 30-50% advance payment with the balance paid before shipment or against copy of shipping documents. MOQs for standard white-label products can start from 500-1,000 units for simpler devices. For highly customized projects, MOQs may be higher (e.g., 5,000-10,000 units) to justify development and tooling costs. Established Indian video laryngoscope manufacturers and suppliers are often negotiable on terms, especially for promising long-term partnerships.
Modern disposable video laryngoscopes from top Indian manufacturers offer core clinical functionality comparable to reusable systems for routine intubations. They provide a clear view of the glottis, adequate illumination, and are ready-to-use. The trade-offs typically come in areas like ultimate image resolution (though this is improving), the feel of a less premium handle material, and the lack of upgradeability. However, for infection control, convenience, and total cost of ownership in high-turnover settings, Indian-made disposable devices present an excellent, cost-effective solution that meets clinical needs.
[1] https://cdsco.gov.in/opencms/opencms/en/Home/
[2] https://www.makeinindia.com/sector/medical-devices
[3] https://www.iso.org/standard/59752.html