







Content Menu
● What Does “Sterile” Mean for Medical Gloves?
● Sterile vs Non-Sterile Medical Gloves
● How Are Medical Gloves Sterilized?
● Manufacturing and Quality Control of Medical Gloves
● Sterile Medical Gloves in Surgery and Invasive Procedures
● Non-Sterile Medical Gloves in Routine Care
● Role of Medical Gloves in Infection Prevention
● Regulatory Standards for Medical Gloves
● Materials Used in Medical Gloves
● Single-Use Nature of Medical Gloves
● How to Choose the Right Medical Gloves
● Common Misconceptions About Medical Gloves
● FAQ
>> 1. Are all medical gloves sterile?
>> 2. When should sterile medical gloves be used?
>> 3. Can medical gloves be reused or re-sterilized?
>> 4. What is the difference between examination and surgical medical gloves?
>> 5. How should healthcare workers choose between latex and nitrile medical gloves?
Medical gloves are not always sterile, and understanding the difference between sterile and non-sterile medical gloves is crucial for safe clinical practice. This article explains when medical gloves are sterile, how they are sterilized, and how healthcare providers should choose the right type for each procedure based on risk level and regulatory standards.
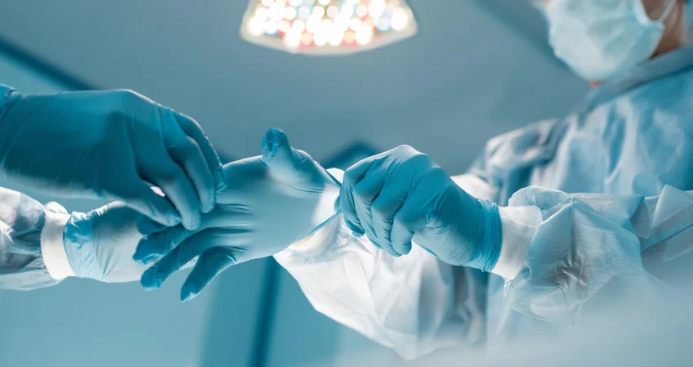
In healthcare, “sterile” means that medical gloves are manufactured and processed so that the probability of any viable microorganism being present is extremely low, typically a sterility assurance level of about one in a million. Sterile medical gloves undergo validated sterilization processes and strict quality control before being sealed in individual sterile packaging. Sterility is maintained only if the medical gloves package remains intact, properly stored, and is opened using aseptic technique at the point of use.
Non-sterile medical gloves, by contrast, are clean and suitable for routine patient care but are not processed to the same level of microbial elimination. They are designed to reduce contamination risk in everyday tasks, such as basic examinations, handling body fluids, or cleaning, but not for invasive procedures entering sterile body sites. Choosing between sterile and non-sterile medical gloves therefore depends on the invasiveness of the procedure and the infection risk for both patient and clinician.
There are two main categories of medical gloves used in clinical environments: examination gloves and surgical gloves. Examination medical gloves can be either sterile or non-sterile, but in many facilities they are predominantly non-sterile, provided in bulk boxes for routine use. These medical gloves protect clinicians and patients from contact with blood, secretions, and potentially infectious materials during general examinations, injections, and basic nursing care.
Surgical medical gloves are almost always sterile, individually packed in pairs, and sized precisely for comfort and dexterity. They are used for operations and invasive procedures where the gloves must form part of a sterile barrier system, such as surgery, interventional endoscopy, or central line insertion. Within each category, medical gloves can be made from different materials such as natural rubber latex, nitrile, or vinyl, each offering specific advantages in terms of elasticity, strength, chemical resistance, and risk of allergy.
Although both sterile and non-sterile medical gloves are considered medical-grade, they serve different purposes. Sterile medical gloves are required when instruments are entering normally sterile areas of the body, including during surgery, childbirth, catheterization of sterile body cavities, or certain endoscopic and interventional procedures. In these contexts, any additional microbial load introduced by medical gloves can significantly increase the risk of surgical site infections or serious complications.
Non-sterile medical gloves are adequate for low-risk contact where the main goal is to prevent cross-contamination between healthcare workers and patients during routine care. They are widely used in outpatient clinics, emergency rooms for non-invasive tasks, and long-term care facilities. In both categories, medical gloves must still meet standards for tensile strength, freedom from holes, and biocompatibility, but only sterile medical gloves undergo terminal sterilization and validation for sterility assurance.
Sterile medical gloves are produced using specific sterilization technologies designed to kill microorganisms without damaging glove materials. Common methods include radiation-based processes such as gamma irradiation or electron beam, and gas-based methods such as ethylene oxide. These techniques penetrate both the packaging and the glove material, inactivating bacteria, viruses, and spores while preserving the integrity and performance of the medical gloves.
The choice of sterilization method depends on the glove material, packaging design, and regulatory requirements. After sterilization, medical gloves are tested to confirm that the validated process achieves the required sterility assurance level, and manufacturers issue batch records and certificates of analysis as evidence. The gloves are then labeled appropriately, with information about sterilization method, lot number, expiration date, and storage conditions, so that hospitals and distributors can trace and manage inventory safely.
The manufacturing process for medical gloves involves several stages to ensure safety and performance. Glove formers are cleaned and then dipped into coagulant and latex, nitrile, or other polymers to form the thin protective film that becomes the glove. After forming, medical gloves are cured to strengthen the material, then washed and leached to remove residual chemicals and, in the case of latex, to reduce soluble proteins that may cause allergies.
Before sterilization, medical gloves are inspected and tested for defects such as pinholes, tears, or uneven thickness. Random sampling and water-leak tests are used to verify that the batch meets a specified Acceptable Quality Level (AQL), which is typically stricter for sterile surgical gloves than for standard examination gloves. Only after meeting these mechanical and barrier performance criteria are medical gloves packaged and sent for sterilization, ensuring that both physical quality and microbiological safety requirements are met.
In surgical environments, sterile medical gloves are a critical component of the sterile field. They protect both patient and surgical team from bi-directional microbial transmission during procedures that expose sterile tissues, open cavities, or implant medical devices. Surgeons often use double-gloving techniques with two layers of sterile medical gloves, improving protection in case the outer glove is punctured during complex or lengthy surgeries.
In other invasive procedures such as central line insertion, bronchoscopy, endotracheal intubation in high-risk cases, or interventional radiology, sterile medical gloves may be required by institutional protocols or guidelines. Wherever a sterile drape field is used and sterile instruments are handled, sterile medical gloves are typically mandated to keep the environment as free from microorganisms as possible. This use is tightly tied to infection prevention strategies and hospital quality metrics.
Outside of the operating room and interventional suites, non-sterile medical gloves are used extensively in everyday patient care. Nurses, physicians, and allied health professionals wear non-sterile medical gloves when drawing blood, starting peripheral IV lines in low-risk settings, performing oral care, or cleaning minor wounds that are not considered surgically sterile fields. These medical gloves help minimize the transmission of pathogens from patient to provider and between patients via healthcare workers' hands.
Non-sterile medical gloves are also widely used for housekeeping tasks in clinical environments, including handling waste, disinfecting surfaces, and processing instruments before sterilization. While they are not sterile, the manufacturing standards ensure they are clean and free of visible contaminants. Healthcare workers should still perform hand hygiene before and after using non-sterile medical gloves, because gloves can have small defects and hands can become contaminated during glove removal.
Medical gloves are one part of a wider infection prevention and control strategy. They are not a substitute for proper hand hygiene, but rather a complement that provides a physical barrier to blood, secretions, and potentially infectious materials. Sterile medical gloves reduce the risk of introducing microorganisms into sterile areas, while non-sterile medical gloves reduce the risk of cross-contamination during routine contact and environmental cleaning.
However, overuse or inappropriate use of medical gloves can paradoxically increase infection risk. For example, wearing the same pair of medical gloves between multiple patients, or touching clean surfaces after touching contaminated materials, can transfer microorganisms. Proper training on when to use medical gloves, how to remove them safely, and when to perform hand hygiene is essential for maximizing their protective benefit and minimizing unintended consequences.

Medical gloves are regulated as medical devices, and manufacturers must comply with international and national standards relating to performance, safety, and sterilization. For sterile surgical medical gloves, specific standards define requirements for design, dimensions, biocompatibility, barrier properties, and packaging. These standards also specify that the gloves must be supplied sterile and that the sterilization process is validated and controlled.
Examination medical gloves, whether sterile or non-sterile, are subject to similar but sometimes slightly less stringent barrier performance criteria compared to surgical gloves. Regulatory agencies also define labeling requirements, including indication of sterility, single-use status, material composition, size, and any latex-allergy warnings. Compliance with these standards ensures that medical gloves from reputable manufacturers provide consistent quality for healthcare providers worldwide.
Medical gloves are manufactured from several types of materials, each with distinct properties. Natural rubber latex has traditionally been favored for its excellent elasticity, tactile sensitivity, and fit, especially in surgical medical gloves where fine motor control is critical. However, latex proteins can trigger allergic reactions in some users and patients, prompting many facilities to transition to latex-free medical gloves when necessary.
Nitrile medical gloves have become a popular alternative because they offer good chemical resistance, durability, and puncture resistance without latex proteins. Vinyl medical gloves, made from PVC, are often used in low-risk settings where high tactile sensitivity is less critical but cost considerations are important. For sterile medical gloves, materials must tolerate the chosen sterilization process without degrading, which is another factor manufacturers must consider during product development.
Virtually all medical gloves, both sterile and non-sterile, are designed and labeled for single use. This means that after being worn for a single patient encounter or procedure, they must be removed and discarded safely. Reprocessing or reusing medical gloves is not recommended, as it can compromise the barrier integrity and increase infection risk. Even if gloves appear intact, microscopic damage or contamination can render them unsafe for further clinical use.
The single-use nature of medical gloves has implications for inventory management and environmental impact. Healthcare facilities must stock adequate quantities of both sterile and non-sterile gloves in different sizes and materials, while also exploring ways to manage waste responsibly. Some manufacturers are researching more sustainable materials and manufacturing processes for medical gloves to reduce the environmental footprint without sacrificing safety.
Selecting the right medical gloves depends on the type of procedure, level of risk, and user needs. For surgical operations and invasive procedures into sterile body sites, sterile surgical medical gloves are required. They should fit snugly, offer excellent tactile sensitivity, and be compatible with the instruments and disinfectants used. In some cases, double gloving is recommended to provide added protection.
For routine examination and non-invasive tasks, non-sterile examination medical gloves are usually sufficient. Facilities should provide different sizes to ensure comfort and dexterity, along with both latex and latex-free options to accommodate allergies. Decision-makers should take into account puncture resistance, chemical resistance, and the potential for allergic reactions when choosing gloves, ensuring that clinical staff are trained to match the right type of medical gloves to each situation.
A frequent misconception is that all medical gloves are sterile simply because they are used in healthcare settings. In reality, only gloves that have undergone validated sterilization processes and are individually packaged as sterile should be considered sterile medical gloves suitable for use in sterile fields. Bulk boxed examination medical gloves are typically non-sterile, even though they are clean and medical-grade.
Another misconception is that wearing medical gloves eliminates the need for hand hygiene. Hands can become contaminated when donning or doffing medical gloves, and glove defects can allow microorganisms to reach the skin. Therefore, proper handwashing or hand antisepsis before and after glove use remains essential for infection prevention. Understanding these nuances helps clinicians use medical gloves effectively as part of a comprehensive infection control program.
Medical gloves are essential protective tools in modern healthcare, but they are not all sterile. Sterile medical gloves, most often surgical gloves, are specially manufactured, sterilized, and packaged to meet stringent sterility assurance standards, making them suitable for surgery and high-risk invasive procedures. Non-sterile examination medical gloves, while still medical-grade, are intended for routine patient contact and environmental tasks where a sterile field is not required.
Understanding the differences between sterile and non-sterile medical gloves, the materials and sterilization methods involved, and the regulatory standards that govern them enables healthcare providers, distributors, and OEM partners to make informed choices. By selecting the right type of medical gloves for each clinical application and using them correctly alongside robust hand hygiene, healthcare organizations can reduce infection risks, improve patient safety, and support high-quality care across all settings.
No, not all medical gloves are sterile. Sterile medical gloves are specifically processed and packaged to meet strict sterility criteria for use in surgery and invasive procedures, while many examination medical gloves are non-sterile and intended for routine care where a sterile field is not required.
Sterile medical gloves should be used for surgical operations and any invasive procedures that enter normally sterile areas of the body, such as major surgery, central line insertion, or certain interventional endoscopic procedures. In these situations, sterile medical gloves help maintain a sterile field and reduce the risk of serious infections.
Medical gloves are designed as single-use devices and should not be reused or re-sterilized once worn. Reuse can damage the glove material, compromise the barrier function, and increase the risk of cross-contamination and infection, even if the gloves appear intact to the naked eye.
Examination medical gloves are typically used for routine patient care and may be non-sterile or sterile, whereas surgical medical gloves are almost always sterile, anatomically fitted, and manufactured to higher standards for strength and tactile sensitivity. Surgical medical gloves are intended for use in operating rooms and high-risk invasive procedures.
The choice between latex and nitrile medical gloves depends on allergy risk, required sensitivity, and chemical exposure. Latex medical gloves offer excellent elasticity and feel but can cause allergic reactions in some users, while nitrile medical gloves provide good durability and chemical resistance without latex proteins, making them a preferred option in many facilities.
[1]https://www.fitonegroup.com/Sterile-Medical-Gloves-application-and-Sterile-Methods-id42540047.html
[2]https://ebeammachine.com/sterile-vs-non-sterile-gloves-key-differences-you-should-know/
[3]https://pmc.ncbi.nlm.nih.gov/articles/PMC12453325/