Views: 222 Author: Lake Publish Time: 2025-11-12 Origin: Site








Content Menu
● Understanding Laryngoscope Blade Types and Materials
● Immediate Post-Procedure Cleaning
>> Transportation to Cleaning Area
>> Mechanical Cleaning Techniques
>> Automated Washer-Disinfectors
● Disinfection and Sterilization
● Inspection and Quality Control
>> Handling and Transportation
● Documentation and Compliance
>> Record Keeping Requirements
>> Regulatory Standards and Guidelines
● FAQ
>> 1. What is the proper sequence for cleaning laryngoscopes blades?
>> 2. Can all types of laryngoscopes blades withstand the same cleaning methods?
>> 3. How can I effectively clean the difficult-to-reach areas of laryngoscopes blades?
>> 4. What are the most common mistakes in cleaning laryngoscopes blades?
>> 5. How often should laryngoscopes blades be replaced?
Laryngoscopes are essential medical devices used in airway management across various clinical settings, from emergency departments to operating rooms. The blades of laryngoscopes represent the component that directly enters the patient's airway, making their proper cleaning and maintenance absolutely critical for infection control and patient safety. These specialized components of laryngoscopes feature complex geometries with hinges, locking mechanisms, and crevices that can harbor organic material and pathogens if not properly cleaned. This comprehensive guide specifically addresses the proper cleaning procedures for laryngoscopes blades, encompassing both immediate point-of-use cleaning and thorough terminal reprocessing protocols.
The importance of correctly cleaning laryngoscopes blades cannot be overstated, as these components directly contact patient tissues and secretions during intubation procedures. Improperly cleaned blades of laryngoscopes can become vectors for healthcare-associated infections and compromise device functionality. Understanding the specific techniques for cleaning different types of laryngoscopes blades ensures both device longevity and optimal patient outcomes, while complying with increasingly stringent regulatory standards for medical device reprocessing. The unique design characteristics of various laryngoscopes blades necessitate specialized cleaning approaches to address their specific challenges.
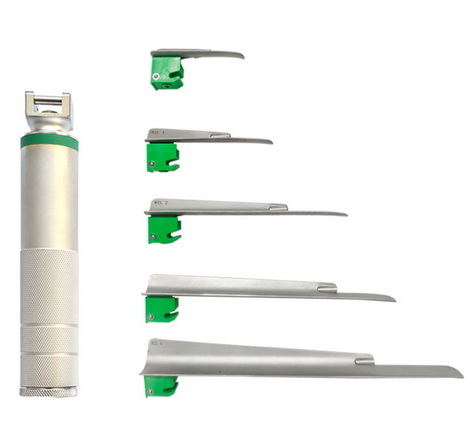
Before addressing cleaning procedures, it's essential to understand the various types of laryngoscopes blades and their material compositions, as these factors significantly influence cleaning requirements. Traditional laryngoscopes blades are typically manufactured from stainless steel, while modern disposable versions may utilize medical-grade plastics. The design variations in laryngoscopes blades—including Macintosh, Miller, Wisconsin, and other specialized shapes—present unique cleaning challenges due to their distinct geometries and surface areas.
The material composition of laryngoscopes blades determines their compatibility with various cleaning agents, temperature tolerances, and durability through repeated reprocessing cycles. Stainless steel blades of laryngoscopes generally withstand more aggressive cleaning methods and higher temperatures, while plastic components may have specific limitations regarding chemical exposure and mechanical cleaning techniques. Understanding these material properties is crucial for developing effective cleaning protocols for laryngoscopes blades that ensure thorough decontamination without compromising device integrity or functionality.
Immediate cleaning of laryngoscopes blades should begin immediately after use at the point of care. This initial step represents the most crucial phase in the reprocessing of laryngoscopes blades, as it prevents the drying of organic material on device surfaces, which can complicate subsequent cleaning steps. Healthcare providers should promptly wipe laryngoscopes blades with a facility-approved disinfectant wipe to remove gross contamination immediately following the intubation procedure. This preliminary cleaning of laryngoscopes blades serves as the foundation for effective terminal reprocessing.
During this initial cleaning of laryngoscopes blades, particular attention should be paid to the distal tip, which has the most direct patient contact, and the locking mechanism, where debris commonly accumulates. The wiping motion should follow the contour of laryngoscopes blades, ensuring all surfaces receive adequate contact with the cleaning agent. Following this immediate cleaning, laryngoscopes blades should be carefully disengaged from handles and placed in designated containers labeled as contaminated, following facility protocols for transport to the central processing area. This prompt attention to laryngoscopes blades significantly enhances the effectiveness of subsequent reprocessing steps.
Proper handling and transportation of contaminated laryngoscopes blades prevents cross-contamination within healthcare facilities. Contaminated laryngoscopes blades should be transported in closed, leak-proof containers that are clearly labeled as biohazardous material. The design of these transport containers for laryngoscopes blades should prevent damage during transit while containing any potential leakage of residual patient materials.
Personnel handling contaminated laryngoscopes blades should wear appropriate personal protective equipment, including gloves and protective eyewear. The transport pathway for laryngoscopes blades from clinical areas to reprocessing locations should be designed to minimize contact with clean surfaces and other personnel. Establishing standardized protocols for transporting laryngoscopes blades ensures consistency and safety throughout this critical phase of the reprocessing cycle.
Thorough manual cleaning represents the cornerstone of effective reprocessing for laryngoscopes blades. Before beginning the cleaning process, reprocessing personnel should don appropriate personal protective equipment, including fluid-resistant gowns, gloves, face shields, and waterproof aprons. The cleaning area for laryngoscopes blades should be prepared with all necessary equipment, including designated sinks, enzymatic detergents, soft-bristled brushes of various sizes, and lint-free cloths.
Laryngoscopes blades should be carefully inspected upon receipt in the cleaning area. Blades showing significant damage, corrosion, or permanent staining should be segregated for potential replacement. The working solution of enzymatic detergent for cleaning laryngoscopes blades should be prepared according to manufacturer instructions, with careful attention to concentration, temperature, and water quality. Proper preparation ensures optimal cleaning efficacy for laryngoscopes blades while preventing chemical damage to the devices.
The mechanical cleaning phase for laryngoscopes blades involves systematic brushing and flushing to remove all organic material and debris. Laryngoscopes blades should be fully submerged in the enzymatic detergent solution and allowed to soak for the manufacturer-recommended time to loosen adhered material. Following soaking, each laryngoscopes blade requires meticulous brushing with appropriately sized soft-bristled brushes specifically designed for these devices.
During mechanical cleaning of laryngoscopes blades, special attention must be paid to several critical areas:
- The locking mechanism and hinge areas, which contain multiple small components and crevices
- The groove along the length of the blade where wiring is typically housed
- The distal tip and light source area, which has direct patient contact
- Any serial numbers or identification markings, which may create additional cleaning challenges
A systematic approach to brushing laryngoscopes blades ensures that all surfaces receive adequate mechanical action. The brushing motion should proceed from the cleanest to the most soiled areas of laryngoscopes blades to prevent redistribution of contamination. Following brushing, laryngoscopes blades require thorough rinsing with clean, preferably filtered, water to remove all detergent residue and loosened contaminants.

Ultrasonic cleaning provides an effective method for removing contaminants from the intricate components of laryngoscopes blades that manual brushing might miss. The cavitation action in ultrasonic cleaners can reach into the smallest crevices of laryngoscopes blades, including the locking mechanisms and hinge areas where biological material commonly accumulates. When using ultrasonic cleaning for laryngoscopes blades, it's essential to follow manufacturer recommendations regarding solution compatibility, exposure time, and energy settings.
Laryngoscopes blades placed in ultrasonic cleaners should be fully disassembled if possible and arranged in specialized baskets to ensure all surfaces are exposed to the cleaning action. The ultrasonic solution should be specifically formulated for medical devices and compatible with the materials used in laryngoscopes blades. Following ultrasonic cleaning, laryngoscopes blades must be thoroughly rinsed with high-quality water and inspected before proceeding to disinfection. Regular maintenance and testing of ultrasonic cleaners ensure optimal performance when processing laryngoscopes blades.
Automated washer-disinfectors offer standardized reprocessing for laryngoscopes blades, reducing variability associated with manual cleaning. These systems provide consistent exposure to cleaning solutions, mechanical action, and rinsing cycles specifically programmed for medical devices like laryngoscopes blades. When using automated systems for laryngoscopes blades, it's crucial to use racks and trays designed specifically for these devices to ensure proper positioning and exposure to cleaning agents.
The programmed cycles for laryngoscopes blades in washer-disinfectors typically include phases for washing, rinsing, and thermal disinfection. Validation of these cycles for specific models of laryngoscopes blades ensures effective reprocessing without device damage. Regular monitoring and maintenance of automated reprocessing equipment used for laryngoscopes blades is essential for consistent results. These systems often provide documentation capabilities that support compliance tracking for laryngoscopes blades reprocessing.
Following thorough cleaning, laryngoscopes blades require high-level disinfection to eliminate pathogenic microorganisms. The choice of disinfectant for laryngoscopes blades depends on material compatibility, efficacy against healthcare-associated pathogens, and facility protocols. Common disinfectants used for laryngoscopes blades include glutaraldehyde, ortho-phthalaldehyde, hydrogen peroxide, and peracetic acid-based solutions.
When chemically disinfecting laryngoscopes blades, strict adherence to manufacturer instructions for both the devices and the disinfecting agents is essential. Key parameters including concentration, temperature, and immersion time must be carefully monitored and documented for each batch of laryngoscopes blades processed. After disinfection, laryngoscopes blades require thorough rinsing with sterile water to remove chemical residues, followed by drying in a controlled environment to prevent recontamination.
For laryngoscopes blades used in sterile fields or on immunocompromised patients, sterilization may be required. Low-temperature sterilization methods such as hydrogen peroxide plasma, ethylene oxide, or vaporized hydrogen peroxide are typically recommended for laryngoscopes blades to prevent damage to components. These methods effectively sterilize laryngoscopes blades without compromising their structural integrity or functionality.
When sterilizing laryngoscopes blades, proper packaging and loading of sterilization chambers ensure effective agent penetration and maintenance of sterility until use. Biological and chemical indicators should be used with each load containing laryngoscopes blades to verify sterilization conditions were met. Strict inventory rotation practices for sterilized laryngoscopes blades prevent outdated devices from entering clinical use, maintaining both sterility and device functionality.
Following reprocessing, every laryngoscopes blade must undergo meticulous visual inspection to verify cleaning efficacy and device integrity. Using adequate lighting and magnification if necessary, reprocessing personnel should examine all surfaces of laryngoscopes blades for residual soil, damage, or wear that might compromise function or cleanability. Particular attention should be paid to the lighting elements, hinges, and locking mechanisms of laryngoscopes blades, where debris commonly accumulates.
During inspection of laryngoscopes blades, any visible residue, discoloration, or damage should prompt return to the cleaning process or removal from service. The visual inspection of laryngoscopes blades represents a critical quality control point that directly impacts patient safety. Documentation of inspection results for laryngoscopes blades provides traceability and supports quality improvement initiatives in reprocessing operations.
Beyond visual inspection, laryngoscopes blades require functional testing to ensure operational reliability. This includes verifying that the locking mechanism engages securely with handles and that the lighting system functions properly when assembled. Functional testing of laryngoscopes blades should simulate clinical use conditions to identify potential failures before devices are needed for patient care.
Documentation of functional testing results for laryngoscopes blades creates a maintenance history that supports predictive replacement strategies. Laryngoscopes blades failing functional tests should be removed from service and referred for repair or replacement according to facility protocols. Regular calibration and maintenance of testing equipment ensure accurate assessment of laryngoscopes blades functionality.
Correct storage of processed laryngoscopes blades preserves their cleanliness and functionality until next use. Laryngoscopes blades should be stored in clean, dry environments protected from dust, moisture, and extreme temperatures. Storage areas for laryngoscopes blades should be designed to prevent crushing, bending, or other physical damage that might compromise device integrity or performance.
The storage method for laryngoscopes blades should protect reprocessed devices from environmental contamination while allowing for adequate air circulation. Organized storage systems for laryngoscopes blades facilitate proper inventory rotation, ensuring older devices are used before newer ones. Clearly labeled storage locations for different types and sizes of laryngoscopes blades prevent confusion and streamline clinical access while maintaining the chain of custody for reprocessed devices.
Proper handling of cleaned laryngoscopes blades maintains their reprocessed state until point of use. Personnel should handle laryngoscopes blades with clean hands or gloves to prevent recontamination. Transportation of laryngoscopes blades within healthcare facilities should utilize covered containers or carts that protect devices from environmental contaminants during transit.
Emergency laryngoscopes blades stored in code carts or rapid response bags require special consideration regarding packaging and regular verification of cleanliness and function. Systems for tracking the location and status of laryngoscopes blades throughout the facility enhance equipment management and ensure availability of properly reprocessed devices when needed. Regular audits of handling practices for laryngoscopes blades identify opportunities for process improvement.
Comprehensive documentation for reprocessing laryngoscopes blades provides traceability and supports regulatory compliance. Records for laryngoscopes blades reprocessing should include cleaning and disinfection or sterilization parameters, lot numbers of chemicals used, and operator identification. This documentation for laryngoscopes blades creates an audit trail that demonstrates adherence to established protocols and manufacturer instructions.
Electronic tracking systems for laryngoscopes blades can automate much of this documentation, integrating with equipment management databases. Maintenance records for laryngoscopes blades, including repairs, part replacements, and performance issues, should be maintained alongside reprocessing documentation. This comprehensive approach to documenting the lifecycle of laryngoscopes blades supports quality assurance programs and regulatory readiness.
Reprocessing of laryngoscopes blades must comply with increasingly stringent regulatory standards from organizations including The Joint Commission, CDC, FDA, and AAMI. These standards provide frameworks for developing reprocessing protocols for laryngoscopes blades that ensure patient safety and device efficacy. Regular review of updated guidelines regarding laryngoscopes blades reprocessing ensures ongoing compliance with evolving standards.
Healthcare facilities should establish clear policies and procedures specific to reprocessing laryngoscopes blades based on device manufacturer instructions and regulatory requirements. Staff education programs for laryngoscopes blades reprocessing should be regularly updated to reflect current standards and best practices. Internal and external auditing of laryngoscopes blades reprocessing practices validates compliance and identifies improvement opportunities.
Proper cleaning of laryngoscopes blades represents a fundamental aspect of infection prevention and patient safety in healthcare settings. The comprehensive approach to cleaning laryngoscopes blades outlined in this guide emphasizes the importance of systematic protocols that address both immediate post-procedure processing and thorough terminal reprocessing. As laryngoscopes blades continue to evolve with material advancements and design innovations, reprocessing methods must adapt to ensure effective cleaning without compromising device functionality.
Healthcare facilities must prioritize adequate training, appropriate resources, and quality monitoring for laryngoscopes blades reprocessing to maintain both device performance and patient safety. The development and implementation of evidence-based protocols for cleaning laryngoscopes blades, coupled with ongoing staff education and compliance monitoring, ensures that these critical airway management components remain safe, functional, and ready for clinical use. Through diligent attention to proper reprocessing techniques for laryngoscopes blades, healthcare providers contribute significantly to reducing healthcare-associated infections and optimizing patient outcomes during airway management procedures.
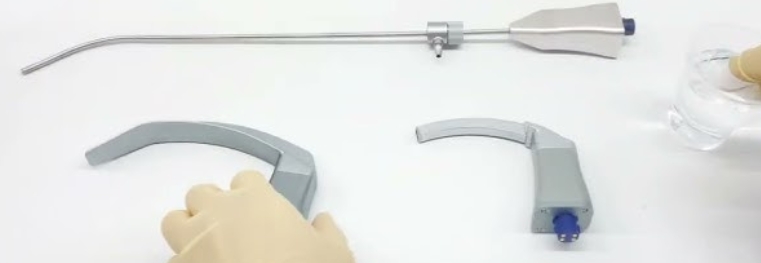
The proper sequence for cleaning laryngoscopes blades begins with immediate point-of-use wiping to remove gross contamination. This is followed by transportation to the reprocessing area in designated containers. The formal cleaning process then proceeds with disassembly (if applicable), immersion in enzymatic detergent, mechanical brushing of all surfaces, thorough rinsing, and inspection. After cleaning, laryngoscopes blades undergo high-level disinfection or sterilization, followed by final rinsing (if chemical disinfection was used), drying, and proper storage. This systematic sequence for laryngoscopes blades ensures thorough decontamination while maintaining device integrity.
Not all laryngoscopes blades can withstand the same cleaning methods due to differences in materials and construction. Stainless steel laryngoscopes blades typically tolerate more aggressive cleaning methods, including higher temperatures and stronger chemical agents. Plastic laryngoscopes blades may have specific limitations regarding chemical compatibility and temperature exposure. Fiberoptic laryngoscopes blades with integrated light sources require special care to prevent damage to delicate components. Always consult the manufacturer's instructions for specific guidance on cleaning each type of laryngoscopes blades to ensure proper reprocessing without device damage.
Effective cleaning of difficult-to-reach areas of laryngoscopes blades requires specialized techniques and tools. The locking mechanism and hinge areas benefit from small, soft-bristled brushes specifically designed for laryngoscopes blades. Ultrasonic cleaning can be particularly effective for removing debris from intricate components of laryngoscopes blades that manual brushing might miss. For channels or grooves in laryngoscopes blades, pressurized flushing with enzymatic detergent may be necessary. A systematic approach to cleaning laryngoscopes blades that addresses each challenging area individually ensures comprehensive decontamination.
The most common mistakes in cleaning laryngoscopes blades include inadequate pre-cleaning at point of use, allowing organic material to dry on surfaces; insufficient brushing of the locking mechanism and hinge areas; using overly abrasive brushes or cleaning tools that damage device surfaces; incomplete rinsing that leaves detergent or disinfectant residues; improper drying that can lead to corrosion or microbial growth; and failing to inspect laryngoscopes blades thoroughly after cleaning. Additionally, using incorrect chemical concentrations or exposure times when cleaning laryngoscopes blades can compromise both cleaning efficacy and device integrity.
The replacement frequency for laryngoscopes blades depends on several factors, including the material composition, frequency of use, and thoroughness of reprocessing. Stainless steel laryngoscopes blades typically have a longer lifespan than plastic versions. Laryngoscopes blades should be replaced when inspection reveals permanent staining, corrosion, cracks, bending, damage to the locking mechanism, or compromised lighting elements. Facilities should establish regular inspection schedules for laryngoscopes blades and maintain documentation of device usage to inform replacement decisions. Manufacturer recommendations regarding the maximum number of reprocessing cycles for laryngoscopes blades should be followed when available.
[1] https://www.cdc.gov/infectioncontrol/guidelines/disinfection)
[2]https://www.aami.org/standards
[3] https://www.fda.gov/medical-devices/reprocessing-medical-devices
[4] https://www.ncbi.nlm.nih.gov/books/NBK553134
[5] https://apic.org/resources/topic-specific-infection-prevention/emergency-medical-services