







Content Menu
● Introduction to Isolation Gowns
● Definition and Primary Purpose of Isolation Gowns
● Types and Classification of Isolation Gowns
>> Disposable vs. Reusable Isolation Gowns
>> ANSI/AAMI PB70 Classification System
● Key Components and Design Features
● Proper Usage and Application Scenarios
>> Standard Precautions Situations
>> Transmission-Based Precautions
>> Specific Clinical Applications
● Standards and Regulations Governing Isolation Gowns
>> 1. What is the difference between an isolation gown and a surgical gown?
>> 2. Can isolation gowns be reused?
>> 3. How do I know what level of isolation gown protection I need?
>> 4. What should I do if an isolation gown becomes visibly soiled during use?
>> 5. Are there specific guidelines for disposing of used isolation gowns?
In the complex landscape of healthcare infection control, the isolation gown stands as a fundamental piece of personal protective equipment (PPE) designed to create a crucial barrier between healthcare workers and potential contaminants. These specialized garments serve as essential tools in breaking the chain of infection transmission in clinical environments. An isolation gown is specifically engineered to protect the wearer's skin and clothing from contact with blood, body fluids, secretions, and excretions during patient care activities. Simultaneously, these gowns help prevent the transfer of microorganisms from healthcare personnel to vulnerable patients, particularly those with compromised immune systems or open wounds.
The significance of the isolation gown in modern healthcare cannot be overstated. These protective garments form an integral component of Standard and Transmission-Based Precautions established by leading health organizations worldwide. Understanding what constitutes an isolation gown, its various types, appropriate usage scenarios, and performance standards is essential for healthcare facilities aiming to maintain the highest levels of patient and worker safety. This comprehensive examination explores the multifaceted nature of isolation gown products, from their basic construction to their critical role in comprehensive infection prevention strategies.

An isolation gown is a protective garment intended to be worn by healthcare personnel during medical procedures and patient care activities to protect both the healthcare worker and the patient from the transfer of microorganisms and body fluids. Unlike a laboratory coat or everyday uniform, an isolation gown is specifically designed as PPE rather than as standard clothing. The fundamental purpose of any isolation gown is to provide an effective barrier that reduces the risk of contamination.
The isolation gown serves multiple protective functions in clinical settings. Primarily, it acts as a shield that prevents potentially infectious materials from reaching the arms and body of the healthcare worker. This protective function is particularly crucial during procedures that may generate splashes or sprays of blood or body fluids. Additionally, the isolation gown helps contain contaminants that might otherwise be transported from one patient area to another on healthcare workers' clothing. This aspect of the isolation gown function is especially important in isolation settings where patients are known to harbor multidrug-resistant organisms or particularly virulent pathogens.
Another critical purpose of the isolation gown is to support compliance with established infection control protocols. The consistent and proper use of isolation gown products according to facility guidelines and situational requirements represents a visible commitment to infection prevention principles. This compliance creates a culture of safety that extends beyond individual protection to encompass broader institutional infection control objectives.
The healthcare industry utilizes various types of isolation gown products classified according to their material composition, barrier protection levels, and intended usage scenarios. Understanding these classifications is essential for appropriate selection and use in different clinical situations.
The most fundamental distinction in isolation gown types lies in their reusability. Disposable isolation gown products are typically constructed from non-woven materials such as polypropylene or polyethylene and are designed for single use before disposal. These isolation gown options offer the advantage of consistent performance characteristics and eliminate the risk of cross-contamination through inadequate laundering. The convenience of disposable isolation gown products has made them increasingly popular in healthcare settings, particularly for procedures with high contamination risk.
Reusable isolation gown products, in contrast, are constructed from woven fabrics, typically cotton or cotton-polyester blends, and are designed to withstand multiple cycles of laundering and sterilization. These isolation gown options must demonstrate durability through repeated processing while maintaining their protective qualities. The decision between disposable and reusable isolation gown products often involves considerations of cost, environmental impact, storage space, and specific clinical application requirements.
The American National Standards Institute/Association for the Advancement of Medical Instrumentation (ANSI/AAMI) PB70 standard establishes a standardized classification system for isolation gown products and other protective apparel based on their liquid barrier performance. This system categorizes isolation gown products into four distinct levels:
- Level 1 isolation gown products offer the lowest level of protection and are suitable for minimal fluid exposure situations, such as during basic patient care or visitors wearing an isolation gown in standard precaution settings.
- Level 2 isolation gown products provide slightly increased barrier protection and are appropriate for low fluid exposure situations, such as during peripheral intravenous line insertion or blood draws.
- Level 3 isolation gown products offer moderate barrier protection for situations with moderate fluid exposure risk, including arterial blood draws, emergency department care, or suturing.
- Level 4 isolation gown products deliver the highest level of liquid and microbial barrier protection and are necessary for high fluid exposure situations, such as major surgical procedures or trauma cases where significant fluid penetration is likely.
This classification system enables healthcare facilities to match the appropriate isolation gown protection level to specific clinical tasks, optimizing both protection and resource utilization.
A well-designed isolation gown incorporates specific features that enhance its protective function, comfort, and ease of use. Understanding these components helps healthcare workers recognize quality in an isolation gown and use it effectively.
The basic construction of an isolation gown includes a body that covers the torso and arms, with openings for the head and hands. Most isolation gown designs incorporate a tie closure system, typically at the neck and waist, though some feature alternative fastening mechanisms such as hook-and-loop closures or snap fasteners. The closure system represents a critical component of the isolation gown as it must secure the garment adequately without creating gaps in protection.
The cuff design represents another important feature of an isolation gown. Various cuff styles exist, including elasticated, knit, and open designs. The isolation gown cuff must interface effectively with gloves to minimize skin exposure at this vulnerable junction. Some isolation gown products feature extended cuffs or thumb loops to help secure the gown sleeve and prevent ride-up during movement.
Additional design elements that may be incorporated into an isolation gown include reinforced areas in zones of high wear or potential fluid exposure, such as the forearms and chest. These reinforced sections enhance the durability and protective capacity of the isolation gown in critical areas. Other features like back coverage options (full or partial), fluid-resistant panels, and breathable materials further refine the isolation gown performance characteristics.
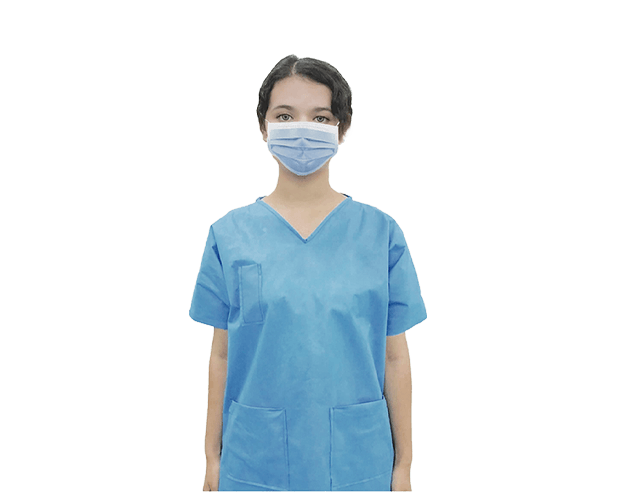
The appropriate use of an isolation gown depends on the specific clinical situation, the anticipated exposure risk, and institutional protocols. Understanding when to don an isolation gown is as important as knowing how to use it correctly.
Under Standard Precautions, healthcare personnel should wear an isolation gown to protect skin and prevent soiling of clothing during procedures and patient care activities that are likely to generate splashes or sprays of blood, body fluids, secretions, or excretions. Donning an isolation gown is appropriate when anticipating direct contact with these substances, regardless of the patient's diagnosis. In such scenarios, the isolation gown serves as a proactive protective measure against unexpected exposures.
The isolation gown assumes even greater importance when Transmission-Based Precautions are implemented. For Contact Precautions, healthcare workers must wear an isolation gown for all interactions that may involve contact with the patient or potentially contaminated areas in the patient's environment. The consistent use of an isolation gown in these situations has been demonstrated to reduce the transmission of multidrug-resistant organisms in healthcare settings.
Beyond precautionary use, specific clinical procedures typically necessitate wearing an isolation gown. These include surgical and sterile procedures, where a sterile isolation gown is required; endotracheal intubation and other respiratory procedures that may generate aerosols; and labor and delivery care where extensive fluid exposure is anticipated. In each scenario, the isolation gown provides tailored protection appropriate to the specific risks involved.
The manufacturing, testing, and classification of isolation gown products are governed by various standards and regulations that ensure consistent performance and safety. These frameworks provide healthcare facilities with objective criteria for selecting appropriate isolation gown products.
In the United States, the Food and Drug Administration (FDA) regulates isolation gown products as medical devices, requiring manufacturers to demonstrate that their products meet specific performance standards. The ANSI/AAMI PB70 standard, formally known as "Liquid Barrier Performance and Classification of Protective Apparel and Drapes Intended for Use in Health Care Facilities," provides the primary classification system for isolation gown barrier protection levels.
The ASTM International (formerly American Society for Testing and Materials) has developed numerous test methods relevant to isolation gown performance, including standards for evaluating resistance to synthetic blood, penetration by blood-borne pathogens, and material strength properties. These standardized tests allow for objective comparison between different isolation gown products.
For reusable isolation gown products, additional standards address durability through repeated laundering cycles. These standards ensure that a reusable isolation gown maintains its protective integrity throughout its intended lifespan. Understanding these regulatory frameworks helps healthcare facilities make informed decisions when selecting an isolation gown for their specific needs.
The isolation gown represents a critical component in the multifaceted approach to infection prevention in healthcare settings. From its basic function as a protective barrier to its role in comprehensive infection control protocols, the proper selection and use of an isolation gown significantly impacts patient and healthcare worker safety. Understanding the different types of isolation gown products, their classification levels, appropriate usage scenarios, and regulatory frameworks enables healthcare facilities to optimize their protective strategies.
As healthcare continues to evolve, so too will isolation gown technology and standards. Ongoing research and development aim to enhance the protective qualities, comfort, and environmental profile of isolation gown products while maintaining their essential safety functions. By staying informed about these developments and adhering to evidence-based practices regarding isolation gown use, healthcare organizations can continue to advance their infection prevention goals and provide the safest possible care environments.
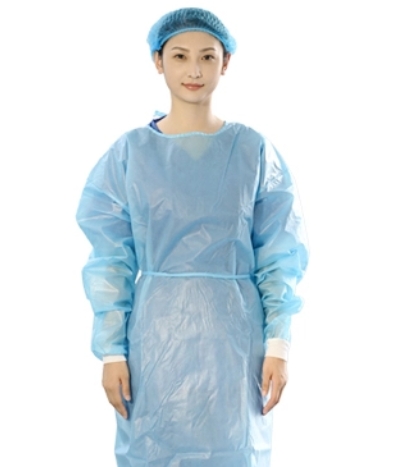
While both are protective garments, a surgical gown is a specific type of isolation gown designed for use in sterile environments like operating rooms. Surgical gowns typically offer higher protection levels (Level 3 or 4) and have stricter sterility requirements compared to standard isolation gown products, which may be used in non-sterile situations with varying protection levels.
Disposable isolation gown products are designed for single use only and should not be reused. Reusable isolation gown products are specifically designed for multiple uses but must be properly laundered and inspected between uses according to manufacturer instructions and facility protocols.
The appropriate isolation gown protection level depends on the anticipated exposure risk during a specific procedure or patient interaction. Healthcare facilities typically establish protocols matching isolation gown levels to clinical tasks based on the ANSI/AAMI PB70 classification system and exposure risk assessments.
If an isolation gown becomes visibly soiled during use, it should be removed as soon as safely possible following proper doffing procedures to prevent self-contamination. Hands should be thoroughly cleaned after removing the soiled isolation gown, and a new gown should be donned if continued patient care is required.
Used isolation gown products should be disposed of according to facility protocols and local regulations. Typically, disposable isolation gown products contaminated with blood or body fluids are treated as medical waste, while those used in standard precautions without visible soiling may be disposed of as regular waste unless facility policy dictates otherwise.
[1] https://www.fda.gov/medical-devices/personal-protective-equipment-infection-control/medical-gowns
[2] https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html
[3] https://www.astm.org/Standards/medical-gowns-standards.html