Views: 222 Author: Lake Publish Time: 2025-10-31 Origin: Site








Content Menu
● Japanese Medical Device Industry Overview
● What Is a Disposable Ureteroscope?
● Advantages of Disposable Ureteroscopes
● Leading Disposable Ureteroscope Manufacturers and Suppliers in Japan
>> Fujifilm Holdings Corporation
>> PENTAX Medical (HOYA Group)
>> Machida Endoscope Co., Ltd.
● Disposable Ureteroscope Manufacturing Process in Japan
● Application and Clinical Use
● Key Features of Japanese Disposable Ureteroscopes
● Engineering Innovation and OEM Advantages
● Export and International Partnerships
● Future Directions and Research
● Frequently Asked Questions (FAQ)
>> 1. What are the primary advantages of using disposable ureteroscopes?
>> 2. Which Japanese companies provide OEM and custom branding for disposable ureteroscopes?
>> 3. How are Japanese disposable ureteroscopes manufactured?
>> 4. What distinguishes Japanese suppliers in the export market?
>> 5. What is the market outlook and innovation trend in Japan?
Japan stands as a leading force in global medical device innovation, particularly recognized for its excellence in Disposable Ureteroscopemanufacturing and OEM services. With an emphasis on precision engineering, regulatory compliance, and rapid deployment, Japanese companies deliver sophisticated urological equipment that sets industry benchmarks worldwide. As international demand for single-use medical devices soars—driven by needs for infection control, cost-effectiveness, and convenience—Japan's Disposable Ureteroscope Manufacturers and Suppliers have become trusted partners for foreign brands, wholesalers, and producers alike.[9][10][11][12]

Japan's medical device sector is founded on principles of technological advancement, quality assurance, and strict adherence to international standards. The country's focus on research and development fosters continuous improvement in endoscopic equipment, including single-use flexible ureteroscopes especially suited for modern urology. Japanese manufacturers consistently achieve ISO-13485 and MDR certification, combining reliability and innovation in each offering. This commitment ensures that every disposable ureteroscope manufactured in Japan meets the needs of both local and global markets, affirming the country's role as a premium exporter of high-end OEM devices.[10][11][13][9]
A disposable ureteroscope is a flexible, single-use endoscopic device designed to access the urinary tract—including the ureters and kidneys—for both diagnostic and therapeutic purposes. Unlike reusable scopes, which must undergo rigorous cleaning between uses, disposable ureteroscopes arrive sterile and ready, significantly lowering the risk of cross-contamination and eliminating costly, time-consuming reprocessing steps. These instruments feature high-definition digital imaging and exceptional maneuverability, enabling precise interventions in challenging clinical situations.[2][4][6][14]
The demand for disposable ureteroscope manufacturers and suppliers in Japan has grown rapidly, based on the following key advantages:
- Reduced infection risk: Single-use design completely eliminates the possibility of cross-patient contamination.[4][14]
- No reprocessing required: Saves time and labor while ensuring consistent device performance.[6][2]
- Superior imaging: State-of-the-art chip-in-tip or CMOS technology offers crystal-clear views of urinary anatomy.[6]
- Cost-effective: Avoids the frequent repair and maintenance costs common with reusable endoscopes.[13][2]
- Global regulatory alignment: Devices are manufactured to ISO, FDA, and MDR requirements with full documentation for export.[11][10]
Thanks to continuous improvement and advanced supply chain management, Japanese manufacturers can fulfill both large OEM projects and specialty batches tailored to evolving clinical needs.[10][11]
Olympus leads the way with cutting-edge flexible video ureteroscope designs, such as the URF-V2 and related product lines, renowned for high-definition imaging and ergonomic operation. Their advanced product lineup is complemented by flexible OEM production, allowing international partners to integrate Olympus technology into their brands with confidence in safety and quality.[15][11][13]
Fujifilm delivers single-use ureteroscopes with integrated digital sensors, using advanced plastics and microelectronics. Their expertise includes not only proprietary devices for the domestic Japanese healthcare market but also expansive OEM programs that support private-label manufacturing for global brands. Their flexible ureteroscopes combine patient safety with ease-of-use and digital connectivity.[5][16][9][10]
As a major player in the field of endoscopic imaging, PENTAX Medical manufactures single-use, flexible ureteroscopes for clinical and OEM customers. Their devices boast digital imaging, wide viewing angles, and extreme flexion—delivered with full international certification for export markets.[17][9][13]
Machida specializes in high-quality flexible and single-use endoscopes, with a strong focus on customized OEM/ODM projects. Their sterilization protocols and imaging solutions set them apart in the global supply chain, catering to both high-volume and specialty requirements from worldwide distributors.[9][11]
Dr.Japan offers full-cycle OEM and ODM services backed by international certifications. Their production capabilities encompass not just disposable ureteroscopes but also a diverse suite of urological and endoscopic disposables, supporting complex customizations and international technical standards.[11][10]
- Karl Storz Japan: Focuses on precision and reliability for high-volume hospital systems.[12][13]
- Medtronic Japan and Smith & Nephew Japan: Deliver additional options for large-scale procurement contracts.[13][9]
- Ambu: Brings unique digital single-use technology to the Japanese market.[14]
- Boston Scientific Japan and Richard Wolf: These global leaders operate comprehensive product and OEM portfolios out of their Japanese offices.[5][13]
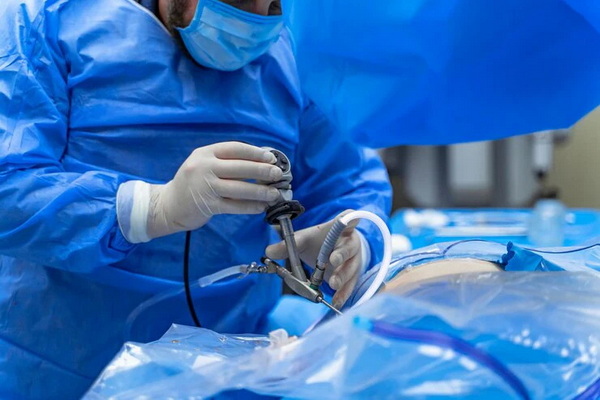
Japanese disposable ureteroscope manufacturing blends the latest in materials science, microengineering, and digital imaging. The process includes:
1. Precision molding of medical-grade plastics to ensure a lightweight, flexible shaft with uniformity and durability.[6]
2. Assembly of integrated digital sensors (often CMOS chips), delivering real-time, high-resolution images.[13][6]
3. Ergonomic and functional design: Handles are engineered for optimal grip and maneuverability, critical during delicate urinary tract procedures.[6]
4. Sterilization and sterile packaging: Devices undergo rigorous ethylene oxide (EO) sterilization before being sealed in medical-grade blister packs—guaranteeing patient safety at the point of care.[11][6]
5. Comprehensive quality assurance, including visual, functional, and sterility testing before the product leaves the facility.[10][11]
With this process, Japanese manufacturers support innovation and quality-needed specifically by foreign OEM customers, ensuring devices are both export compliant and ready for immediate clinical use.
Disposable ureteroscopes are indispensable for addressing upper urinary tract disease, particularly kidney stones and strictures. These devices allow urologists to navigate complex anatomy, apply energy for lithotripsy, and retrieve debris—all with a single, sterile instrument. The simplicity of single-use devices reduces setup time in the operating room, minimizing delays and downtime between procedures.[18][2][14][6]
Japan's disposable ureteroscope market has shown strong, sustained growth, with revenues climbing from USD 13 million in 2024 and projected to reach USD 16 million by 2030. Flexible ureteroscopes dominate the segment, accounting for over 90% share, thanks to their adaptability across a wide range of applications and patient populations. The country is increasingly recognized as a strategic source for global procurement, with major international brands and partners relying on Japanese OEMs for advanced device supply.[5][10]
Drivers for market growth include:
- Heightened awareness of infection control and regulatory mandates
- Technological advances in chip imaging and digital integration
- Greater frequency of kidney stone disease globally
- Expansion of export logistics and OEM manufacturing in Japan
- Flexible shaft (often 8.5 Fr outer diameter; working length up to 670 mm)[6]
- 275° up/down deflection, wide 120° field of view
- CMOS chip or “chip-in-tip” imaging for true-color visualization[6]
- Integrated LED or fiber-optic light source
- 3.6 Fr working channel compatible with baskets, lasers, and stents[6]
- EO sterilized and individually blister-packed for safety
These features foster procedural excellence and efficiency, particularly under demanding clinical conditions.
Japanese engineering delivers not only robust product quality but also:
- Rapid OEM development cycles for custom branding and regulatory needs
- Batch flexibility (from small to very large orders) for both niche and mainstream markets
- Comprehensive documentation and after-sales support for international clients[10][11]
Japanese suppliers continuously refine their R&D, closely collaborating with clinicians and global brand owners, ensuring that next-generation devices emerge ahead of regulatory changes and clinical trends.
Japan leads the region in exporting premium disposable ureteroscopes, covering North America, Europe, Asia-Pacific, and the Middle East. Foreign customers benefit from:[11][10]
- International shipment via validated temperature- and humidity-stable packaging
- FDA, MDR, ISO, and other regulatory approvals
- Detailed technical specifications for seamless integration with local hospital systems
- Sales and support in multiple languages[19][10][11]
Japanese manufacturers are known for transparent supplier communications, technical consultation, and robust logistics management, positioning them as ideal OEM partners for new private-label ventures and established medical brands alike.
Japan's focus on digitalization, AI-driven imaging analysis, and minimal access urology will continue driving disposable ureteroscope innovations. The country's commitment to sustainability also suggests impending adoption of recyclable materials and eco-conscious production protocols. With ongoing clinical studies confirming the safety, efficacy, and cost advantages of single-use devices over conventional reuse, demand for high-performance disposable ureteroscope manufacturers and suppliers from Japan is set to accelerate worldwide.[2][4]
Japanese disposable ureteroscope manufacturers and suppliers are synonymous with technological leadership, quality, and reliability in the global medical device market. Through robust OEM services, regulatory excellence, and custom production capabilities, these companies empower overseas partners to meet stringent clinical and market demands. By sourcing disposable ureteroscopes from Japan, brands, wholesalers, and producers secure a competitive edge in both product innovation and patient care.
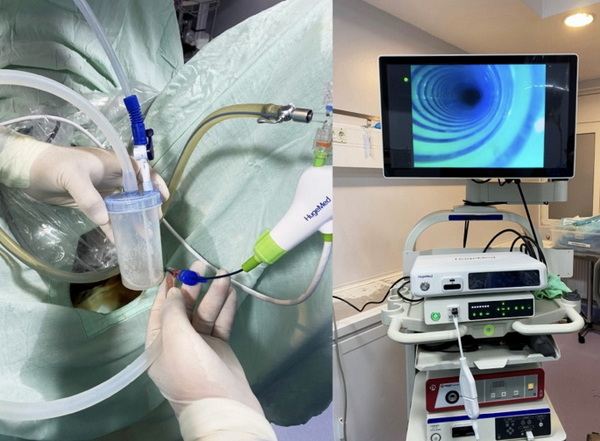
Disposable ureteroscopes greatly reduce infection risks, eliminate the need for device reprocessing, and lower long-term costs by avoiding expensive repairs. They also offer consistently high imaging quality.[4][14][2]
Leading OEM providers include Olympus, Fujifilm, PENTAX Medical, Machida Endoscope, and Dr.Japan. Each offers tailored production and global export documentation.[13][10][11]
Production combines precision medical plastics molding, digital chip integration, ergonomic design, EO sterilization, and thorough post-manufacture quality testing, resulting in high-standard, export-ready devices.[11][6]
Japanese manufacturers deliver international regulatory compliance, efficient batch customization, extensive documentation, and strong after-sales support. Foreign partners benefit from fast and secure logistics with full technical backing.[19][10][11]
Japan's disposable ureteroscope market is expected to grow steadily through 2030, led by ongoing improvements in digital imaging, device miniaturization, and environmentally sustainable manufacturing.[2][4][5]
[1](https://www.linkedin.com/pulse/japan-single-use-flexible-video-ureteroscope-urf-v-0qbse)
[2](https://pmc.ncbi.nlm.nih.gov/articles/PMC8784383/)
[3](https://www.gminsights.com/industry-analysis/disposable-ureteroscope-market)
[4](https://www.sciencedirect.com/science/article/pii/S0195670125002415)
[5](https://www.grandviewresearch.com/horizon/outlook/disposable-ureteroscope-market/japan)
[6](https://advinhealthcare.com/flexible-single-use-ureteroscope-hassle-free-flexibility-every-procedure/)
[7](https://www.archivemarketresearch.com/reports/single-use-flexible-ureteroscope-539475)
[8](https://www.openpr.com/news/4175501/disposable-ureteroscope-market-evolution-by-2032-disruptive)
[9](https://www.mordorintelligence.com/industry-reports/japan-endoscopy-devices-market/companies)
[10](https://japanesemfg.com/insights/medical-hygiene-consumables/)
[11](https://www.drjapan-jp.com/en/oem-odm)
[12](https://v-mr.biz/disposable-ureteroscope-market)
[13](https://www.grandviewresearch.com/industry-analysis/disposable-ureteroscope-market-report)
[14](https://www.ambu.com/endoscopy/urology/products)
[15](https://sites.google.com/view/pixel-prism/home/market-research-collective/japan-single-use-flexible-video-ureteroscope-urf-v-market-by-application)
[16](https://bimedis.com/manufacturers/japan/endoscopy-equipment)
[17](https://www.reportprime.com/ureteroscopes-r19437/company)
[18](https://www.nature.com/articles/s41598-025-00515-3)
[19](https://www.volza.com/p/medical-disposables/export/export-from-united-states/cod-japan/)